Innate immunity is the first line of defense against infection with a pathogen. In the case of a viral infection, virally-derived nucleic acids are detected in the cytosol of infected cells by DNA sensors and RNA sensors (known as RIG-I like receptors). Activation of these receptors triggers the production and secretion of type I interferons by many cell types. Interferons activate the interferon receptor (IFNAR) and this induces an antiviral state and the expression of hundreds of interferon-stimulated genes (ISGs), which restrict viral replication in many ways.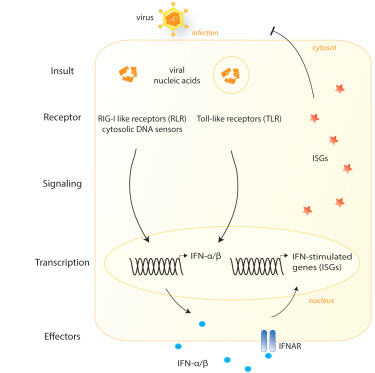
Nucleic acids, however, are integral components of our own cells and in some cases our own DNA and RNA molecules accidentally trigger the nucleic acid sensing machinery. This leads to a type I interferon response in the absence of an infection, i.e. sterile inflammation. This is seen, for example, in patients who suffer from type I interferonopathies, a group of severe diseases caused by single gene defects that lead to chronic and excessive interferon production. Such patients would benefit from treatments that interfere with interferon production.
Conversely, type I interferon production by tumor cells or tumor-infiltrating immune cells can improve disease outcome. Type I interferons can contribute to immune control of cancer by recruiting effector cells and by promoting antigen-cross presentation by dendritic cells. An intra-tumoral interferon signature often correlates with a better prognosis and a better response to different types of therapies. However, interferon production in tumors is often suboptimal. Therefore, strategies to boost intra-tumoral interferon responses hold great potential to improve cancer therapy.
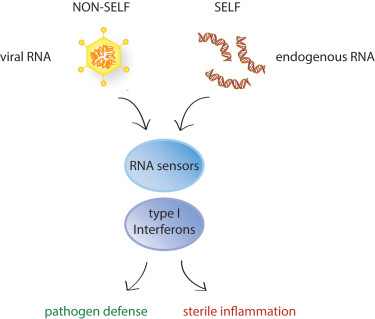
How type I interferons are induced in these different disease contexts is largely unknown and the focus of our research. What type of endogenous RNA molecules activate RNA sensors? What mechanisms do cells have in place to prevent this? We are using novel methods, such as iCLIP (individual-nucleotide UV crosslinking and immunoprecipitation) and CRISPR/Cas9, to understand the molecular basis of self/non-self recognition by the nucleic acid sensors of the innate immune system. The goal of our research is identify novel strategies to steer sterile inflammatory responses into a certain direction and to use it to our own advantage.
Selection of our ongoing projects:
- Defects in nucleic acid sensing lead to impaired pathogen defense. Conversely, inappropriate activation of nucleic acid sensors leads to unwanted and excessive production of type I interferons and pro-inflammatory cytokines and ultimately immunopathology. The nucleic acid sensing machinery therefore requires tight control. Our group is investigating how RNA sensing is controlled by post-translational modifications. In addition, we have identified proteins that are important regulators of RNA sensing during viral infection and in the context of sterile inflammation. We are currently investigating the precise mechanism by which these regulators control the nucleic acid sensing machinery.
- The RNA editing enzyme ADAR1 acts as a gatekeeper of spontaneous type I IFN production. ADAR1 deaminates adenosines and converts these into inosines. A-to-I editing of self RNA is essential to prevent the unwanted recognition of our own RNA by the RNA sensors MDA5 and LGP2. In addition, ADAR1 prevents activation of PKR and ZBP1, which control proliferation and cell death. Our group investigates the consequences of ADAR1 deficiency at a molecular level. Furthermore, we are investigating other proteins that function as gatekeepers of type I IFN production, akin ADAR1.
- The RNA helicase LGP2 is a RIG-I-like receptor that is not well understood. LGP2 can potentiate MDA5 signaling and inhibit activation of RIG-I. However, the exact mechanism through which LGP2 mediates these effects, its alternative functions, and its mode of regulation require further elucidation. Our group investigates the role of LGP2 in viral and self RNA sensing.
- Non-coding RNAs may regulate pathogen defense during viral infection. They may directly impact on replication of a virus or they may regulate how the innate immune system responds to the invader. We are currently investigating how certain non-coding RNAs control viral infection.