Our research focusses on the regulation of T cell immunity and use this knowledge to improve immunotherapy.
Decoding T cell immunity
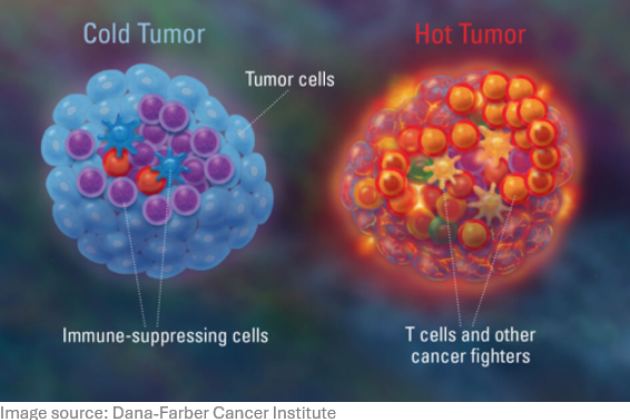
T cells play a central role in the control of viral infections and cancer. Moreover, the capacity of T cells with different properties is of major interest for its usage in vaccines and immunotherapeutic approaches such as immune checkpoint therapy and adoptive T cell therapy. We study how costimulatory and inhibitory molecules cooperate and contribute to the differential programming of T cells resulting in expansion and differentiation into various effector and memory subsets. Our research contains the dissection of T cell fitness properties at the molecular and cellular level including cytokine polyfunctionality, cytotoxicity and metabolic requirements.
Understanding and improving T cell based immunotherapy
We aim to understand and improve immunotherapy by integrating the knowledge of the programming of T cells to improve the fitness of T cells. The investigations comprise the study of disease- and therapy-responsive T cells in infectious disease and cancer. Researched immunotherapies include (therapeutic) vaccines, immune checkpoint modulators, chemo-immunotherapy and adoptive T cell therapy.
Platforms and technologies
Both themes cover the spectrum from molecular and gene editing technology in vitro/ex vivo to in vivo experimental models for human disease and the use of human samples.
We employ a unique combination of platforms and models: human ex vivo assays, experimental tumor models (melanoma, colon cancer, sarcoma, prostate cancer, ovarian cancer, breast cancer and lung cancer), and experimental infection models including mouse cytomegalovirus (MCMV), vaccinia virus (VV), lymphocytic choriomeningitis virus (LCMV), Listeria monocytogenes.
For the in-depth characterization of the immune response on a molecular and cellular level we use (single-cell) profiling technologies including spectral flow cytometry, (single-cell) RNA sequencing, proteomics, and metabolomics. To allow exploration of the biological markers and parameters that relate to immunotherapy, we use gain-of-function and loss-of-function tools such as transgenic and knockout mice, CRISPR-Cas gene editing, immunomodulating antibodies, and MHC multimer technology.
Contact R.Arens@lumc.nl for more information and collaborations.