Chemistry is the science of connecting atoms to generate newly designed molecules, and understanding their properties and interactions. Chemical Immunology connects chemistry with the intriguing complexity of immunology. It is our ambition to bring together and motivate people from various disciplines to make a difference in the field of immunology and to connect chemistry and biology in our teaching activities.
Targeted immunomodulation
The immune system is regulated via many molecular cues, checks and balances. Providing the correct set of cues at the right time to the right subset of immune cells in the correct environment is crucial to achieve the envisioned immune response. In many immune-mediated pathologies such checks and balances are hijacked contributing to local disease initiation and progression. Systemic counteraction of these hijacks often results in severe immune-related adverse events (irAEs). Molecular targeting approaches enable local immunomodulation while minimizing the risk of systemic irAEs.
Cancer could be considered an immune-mediated disease. Tumors hijack immunological control mechanisms such as immune checkpoints to prevent activation and cancer cell eradication by T cells. We aim to develop strategies for chemistry-based precision immunotherapy using small molecules, nanomaterials and antibody targeted approaches to i) induce antigen-specific immune responses, ii) to modulate specific immune cells, such as macrophages in the tumor microenvironment and iii) to monitor specific immune cell phenotype and function in vitro and in vivo.
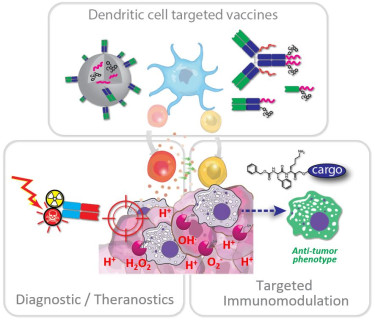
We develop DC targeted vaccines to deliver tumor antigen in combination with immune activating adjuvants to specific DC subsets. Besides nano-formulations for crucial co-delivery of these molecular cues we are also developing antibody- and nanobody-based single-molecule conjugate vaccines. To achieve this we have established a CRISPR/HDR hybridoma engineering platform to produce antibody-derived products with desirable properties, such as chimeric and optimized mutant isotypes, as well as tags for site-specific modification. In conjunction we aim to develop molecules and materials responsive to chemical cues present in the immunosuppressive tumor microenvironment to achieve local immunomodulation to prime the tumor for the infiltration and activation of tumor specific effector immune cells, such as cytotoxic T cells which have been induced by our DC targeted vaccines. To study the effect of these immunotherapeutic interventions we develop antibody- and small molecule-based multimodal, multiscale and ‘smart’ imaging tools.