Our research group focuses on the development of therapeutic T cell vaccines and local immunotherapeutic delivery strategies, aiming to enhance the effectiveness of cancer immunotherapy while reducing systemic side effects. Our approach is twofold: we target dendritic cells in the skin to prime T cell responses and we reprogram suppressed T cells within the tumor microenvironment through local delivery of immunomodulators. Our technologies are on the interphase of immunology, pharmaceutical sciences and engineering.
1. Therapeutic T cell vaccine development
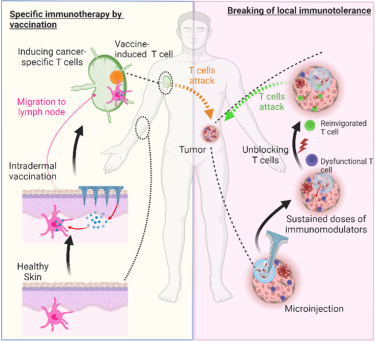
We aim to induce new cancer-specific T cells via vaccination strategies that target dendritic cells (DCs), which are essential for initiating robust T cell responses. Our current focus is on two synergistic components:
i) Targeting the skin
The skin is a highly immunocompetent organ densely populated with DCs. By delivering vaccines directly into the skin, we aim to improve antigen presentation and enhance systemic T cell activation. We design and evaluate needle-free delivery systems, such as dissolvable microarray patches (MAPs) and jet injectors to ensure precise, minimally invasive intradermal administration. These methods can improve immunogenicity, reduce antigen dose requirements, and increase patient comfort (i.e., pain-free delivery and self-administration).
ii) Nanoparticulate vaccine formulations
We formulate molecularly-defined antigens, such as peptides and DNA/mRNA, into nanoparticles—particularly liposomes and PLGA—to promote uptake by dendritic cells. To enable clinical translation, we focus on the development of scalable GMP-compliant formulation methods that encapsulate vaccine antigens into thermostable formulations.
2. Local immunomodulation of the tumor microenvironment
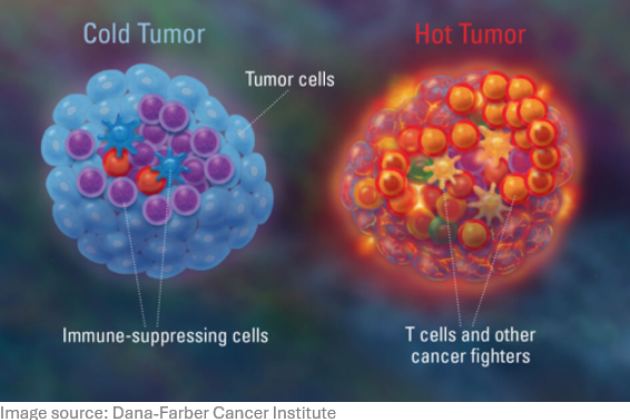
T cells present in the tumor microenvironment (TME) are often suppressed by local immune regulatory mechanisms. Systemic immunotherapies such as checkpoint inhibitors can restore T cell function, but carry the risk of severe immune-related adverse events. Our goal is to develop technologies that overcome immune suppression locally, within the tumor, by delivering immunomodulators directly to the TME.
i) Precision intratumoral delivery
We are developing injection technologies—including glass fiber-based microinjection systems—for the delivery of nanoliter-scale drug formulations directly into tumors. These systems are designed to ensure high spatial precision, minimal tissue damage, and maximal local effect.
ii) Controlled-release immunotherapeutic formulations
We design slow-release formulations containing cytokines and other immunomodulatory agents to retain high local drug concentrations while reducing dosing frequency and systemic exposure. We are exploring this strategy to reshape the TME and restore local immune control while minimizing toxicity.